Have you ever wondered why food cooks faster in a pressure cooker than a regular pot? Or why some chemical reactions happen in a flash while others take years to complete? The answer lies in the fascinating world of reaction kinetics, where we explore the factors that influence the speed at which chemical reactions occur. In this comprehensive lab report, we’ll delve into the science behind these reactions, uncovering the critical factors that determine how quickly reactants transform into products.

Image: myriverside.sd43.bc.ca
Understanding reaction rates is crucial in various fields, from chemistry and biology to medicine and environmental science. By manipulating these rates, scientists can optimize industrial processes, design new drugs, and even predict the environmental impact of chemical reactions. This report aims to provide a clear and accessible guide to understanding the factors that govern reaction rates, making you a more informed and curious observer of the chemical world around you.
The Essence of Reaction Rate
At its core, the **rate of a chemical reaction** represents how quickly reactants are consumed and products are formed. It’s measured as the change in concentration of a reactant or product over a specific time interval. Imagine a crowded dance floor—the faster the people dance, the quicker the floor empties. Similarly, the faster a chemical reaction proceeds, the quicker the reactants disappear and the products appear.
Key Determinants of Reaction Rate: The Influential Factors
Imagine a complex dance routine involving multiple partners. The speed at which the choreography unfolds depends on factors like the dancers’ skill, the complexity of the steps, and the space they have to move around. Similarly, the rate of a chemical reaction is influenced by several key factors, each playing a critical role in determining its speed.
1. Concentration: The More the Merrier (Usually)
Just like increasing the number of dancers in a room leads to more collisions and interactions, increasing the concentration of reactants in a chemical reaction enhances the frequency of collisions between them. These collisions are essential for breaking existing bonds and forming new ones, leading to product formation. Generally, higher concentrations translate to faster reaction rates.
Example: A classic example is the reaction between hydrochloric acid (HCl) and magnesium (Mg) to produce hydrogen gas (H2). As you increase the concentration of HCl, more acid molecules collide with the magnesium surface, accelerating the production of hydrogen gas and resulting in a faster reaction rate.
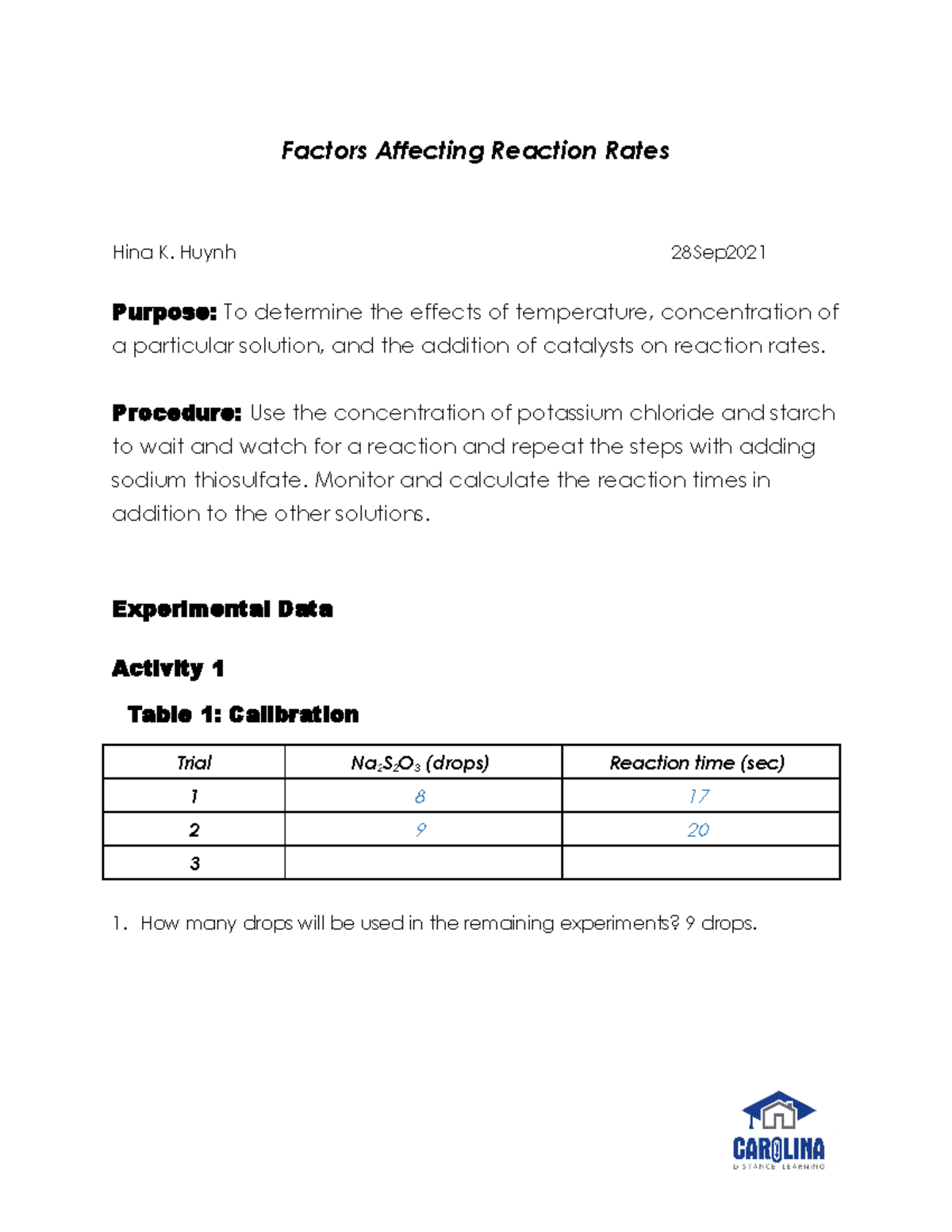
Image: www.studocu.com
2. Temperature: Heat Things Up
Think of a room full of people—the warmer it gets, the more active and energetic they become, leading to increased movement and interactions. Similarly, an increase in temperature provides reactants with more kinetic energy, making the molecules move faster and collide more forcefully. These energetic collisions increase the likelihood of breaking bonds and forming products, boosting the reaction rate.
Example: Cooking is a familiar example of temperature’s impact on reaction rate. If you want to boil water (H2O) faster, you simply increase the temperature of the stovetop. The higher temperature speeds up the water molecules’ movement, accelerating the process of reaching the boiling point.
3. Surface Area: Break It Down for Faster Reactions
Imagine a giant cake—it takes a lot of time to cut into small pieces with a knife. However, if you break it down into smaller chunks, it’s much easier and faster to consume. The same principle applies to chemical reactions. Increasing the surface area of a reactant provides more locations for collisions to occur, effectively accelerating the reaction rate.
Example: Take the example of a piece of iron rusting in the air. Rusting is a chemical reaction between iron (Fe) and oxygen (O2) in the presence of moisture. If you have a large piece of iron, the reaction is slow because only the surface area is exposed to air. However, if you cut the iron into smaller pieces, significantly increasing its total surface area, the reaction rate dramatically increases, leading to faster rusting.
4. Catalysts: The Reaction Accelerators
Think of a skilled dance instructor—they can guide the dancers to perform the moves more efficiently and effectively. Similarly, catalysts act as “reaction enhancers.” They provide an alternative pathway for the reaction to proceed, lowering the activation energy needed to initiate the process. Catalysts don’t get consumed in the reaction but help the reactants transform into products more quickly.
Example: Enzymes are biological catalysts that accelerate countless biochemical reactions within our bodies. For instance, the enzyme lactase helps break down lactose, a sugar found in milk. Without lactase, lactose digestion would be incredibly slow, causing lactose intolerance.
5. Nature of Reactants: The Key Players
The specific chemical nature of the reactants plays a significant role in determining reaction rate. Some molecules readily react, while others are relatively inert. The strength of bonds within reactants and the types of bonds that need to be broken or formed during the reaction are important factors.
Example: Sodium (Na) reacts violently with water (H2O), generating a lot of heat and producing hydrogen gas. However, gold (Au) is relatively unreactive and doesn’t readily react with most substances under normal conditions. These differences in reactivity are due to the inherent properties of the atoms and the types of bonds they form.
Experimental Investigation: Measuring Reaction Rates in the Lab
To gain a deeper understanding of reaction kinetics, conducting experiments in a lab setting is crucial. By systematically varying the factors discussed above, we can measure the resulting changes in reaction rate and establish quantitative relationships.
Typical lab experiments often involve measuring the time required for a specific amount of reactant to be consumed or the time taken for a specific amount of product to be formed. Common techniques include:
- Colorimetry: Measuring the change in color intensity of the solution as the reaction progresses. This method works especially well for reactions involving colored reactants or products.
- Gas Collection: Measuring the volume of gas evolved as a product of the reaction.
- Conductivity Measurement: Measuring the change in electrical conductivity of the solution as a reaction progresses. This is particularly useful for reactions involving the formation of ions.
Through careful analysis of the experimental data, we can derive mathematical models that describe the relationship between reaction rate and the factors influencing it. This allows for accurate prediction of reaction behavior under different conditions and lays the foundation for optimizing reaction processes in various applications.
Real-World Implications of Reaction Rate Control
Understanding and manipulating reaction rates has far-reaching implications across multiple industries and scientific disciplines.
- Industrial Chemistry: Optimizing reaction rates is crucial for maximizing production efficiency and reducing waste. For example, in the production of ammonia (NH3) for fertilizer, careful control of temperature, pressure, and the use of catalysts is essential for efficient synthesis.
- Pharmaceutical Industry: Understanding reaction rates is critical for designing and developing new drugs. Drug synthesis involves intricate multi-step reactions, and controlling these steps is vital for ensuring product quality and minimizing side effects.
- Environmental Science: Reaction rates play a significant role in understanding and managing environmental pollution. For instance, the rates of degradation of pollutants in the environment depend heavily on factors like temperature, pH, and the presence of microorganisms.
- Food Industry: Reaction rates are vital for ensuring food safety and quality. Chemical reactions play a role in food spoilage, and controlling these reactions through appropriate preservation techniques like refrigeration or sterilization is essential.
Factors Affecting Rate Of Chemical Reaction Lab Report
Conclusion: Embracing the Dynamics of Chemical Reactions
The world of chemical reactions is a fascinating dance of molecules, driven by factors like concentration, temperature, surface area, catalysts, and the inherent nature of the reactants themselves. By understanding these factors, we gain an unparalleled ability to control and optimize the rate at which these reactions occur, leading to advancements in various fields from manufacturing and medicine to food science and environmental protection. So, the next time you encounter a chemical reaction, take a moment to appreciate the intricate interplay of factors that govern its speed. It’s a testament to the dynamic nature of our chemical world, where a multitude of factors work together in a delicate ballet to create the products we rely upon.
This lab report has only scratched the surface of the vast and exciting world of reaction kinetics. To delve deeper into this captivating field, explore additional resources like textbooks, scientific journals, and online platforms. And remember, the journey of scientific discovery is ongoing. Share your experiences, ask questions, and contribute to our growing understanding of the chemical universe—a universe full of fascinating reactions unfolding right before our eyes.






