Imagine a bustling city, filled with people rushing about. The more people there are, the more frantic their movements. Now picture that city in winter, a chill in the air making people huddle together, moving more slowly. This analogy helps visualize the core principle of how temperature affects the movement of particles. It’s all about motion, a dance of tiny entities that dictate the world around us!
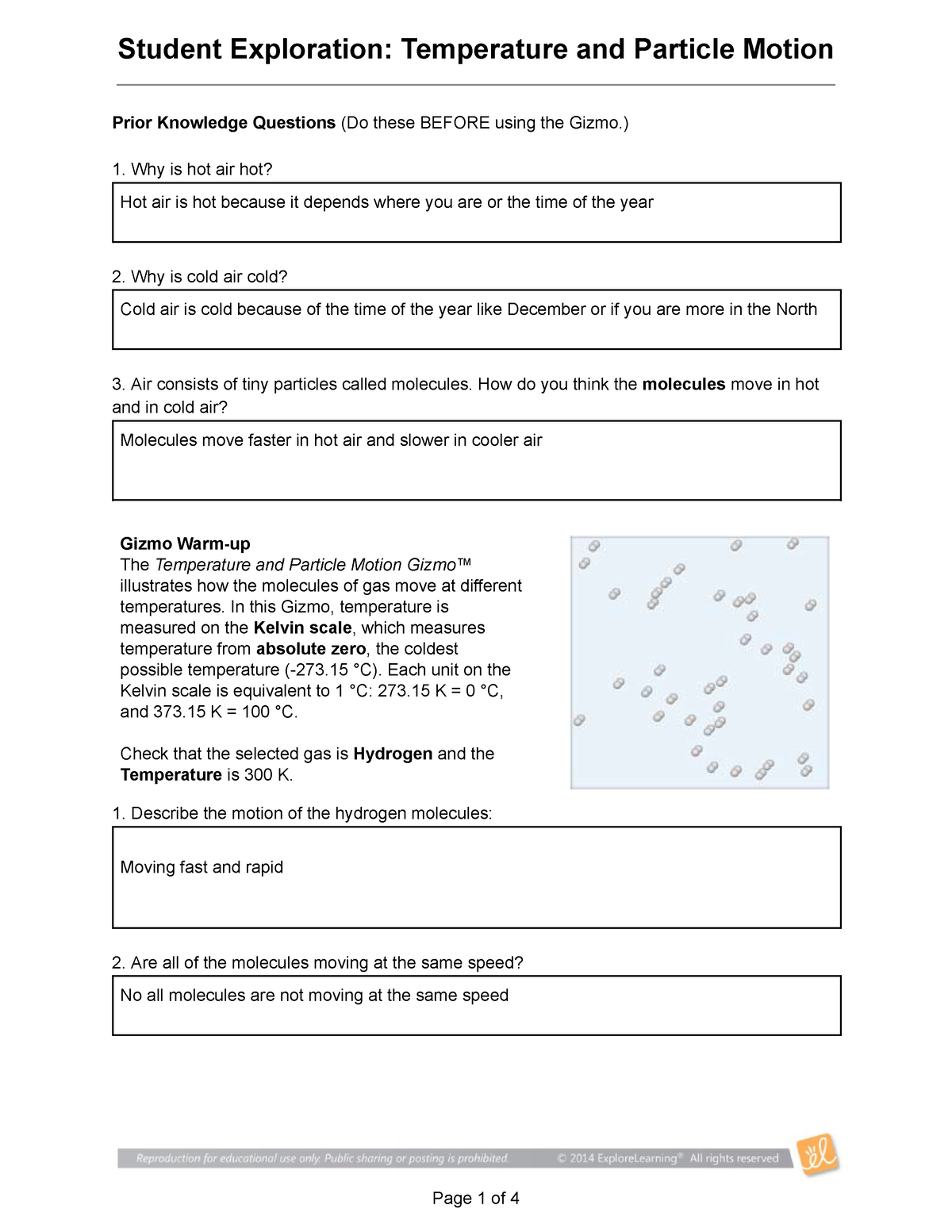
Image: www.studocu.com
In this journey, we’ll explore the fascinating relationship between temperature and particle motion. We will dive into the “Gizmo” world, a virtual playground where we can manipulate the world of tiny particles. By manipulating temperature, we can witness firsthand the effect it has on the behavior of matter. From the quiet stillness of frozen ice to the frenzied vibrations of boiling water, the movement of particles tells a story, a story that science helps us decode.
A Peek into the Invisible World: Understanding Particle Motion
Everything around us, from the air we breathe to the chair you are sitting on, is made up of tiny particles called atoms and molecules. These are so small that we can’t see them even with the most powerful microscopes. But they are constantly in motion, a whirlwind of vibrant activity.
This constant movement of particles is called kinetic energy. The hotter an object is, the faster these particles are moving. Think of it like this: when you heat up a pot of water, you are adding energy to the water molecules. This energy makes them jiggle and shake with more vigor, causing them to move further apart. As they move faster, they bump into each other more frequently, creating the sensation of heat.
The Gizmo: A Visual Playground for Particle Motion
The “Gizmo” is a revolutionary tool for learning about particle motion. Imagine a virtual laboratory where you can manipulate temperature, observe its effects on different states of matter, and even control the properties of the particles themselves. This interactive environment allows us to see the invisible, witnessing the dance of particles in a way that traditional textbooks could never achieve.
Temperature’s Grip: Controlling the Motion of Particles
Temperature is like a conductor, directing the energy and movement of particles. When you raise the temperature of a substance, you are essentially injecting it with more energy. This causes the particles to speed up, dance more frantically, and spread further apart. The increased distance between particles is why solids melt to form liquids, and liquids evaporate to become gases.
Let’s take a closer look at the three states of matter and how temperature influences particle motion:
Solids: In a solid, the particles are tightly packed together and vibrate in fixed positions. They have minimal freedom to move around. Think of soldiers in a formation, standing close together, but allowed to jiggle their legs or arms. The lower the temperature, the less they move, like soldiers standing stiffly at attention.
Liquids: In a liquid, the particles have more energy and freedom, allowing them to move around and slide past each other. It’s like the soldiers in our formation now have more space to move, allowing them to move around a bit, bumping into each other, but still relatively close together.
Gases: In a gas, the particles are far apart and move with the most freedom. They are like soldiers completely free to run around, bumping into each other occasionally. As temperature increases, the particles move even faster, constantly colliding with each other and with the walls of their container.
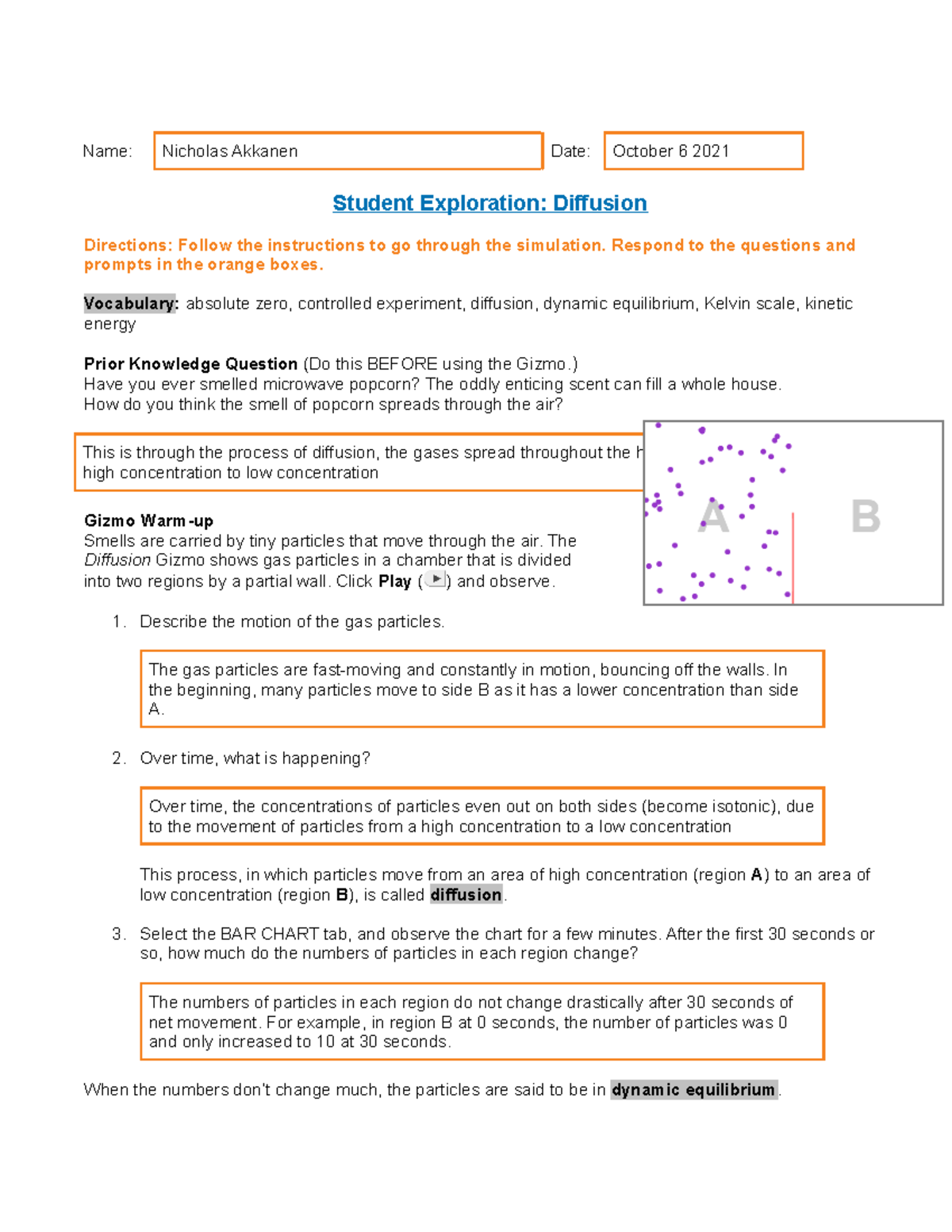
Image: www.studocu.com
The Gizmo’s Power: Understanding Real-World Phenomena
The Gizmo provides a powerful tool to understand everyday phenomena. For instance, have you ever wondered how a hot air balloon floats? It’s all about particle motion! The heat from the burner causes the air inside the balloon to expand, making the particles move faster and further apart. This hot air is less dense than the surrounding cold air, creating an upward force that lifts the balloon.
The Gizmo: A Gateway to Exploration
The “Gizmo” not only makes learning about particles engaging and intuitive, but it can also help us understand real-world applications. It can help us visualize the principles behind phenomena such as diffusion, osmosis, or even the workings of a refrigerator. It’s a gateway to unlocking the mysteries of the microscopic world, a world that profoundly shapes our everyday experiences.
Learning from the Gizmo: Practical Takeaways
Here are some key takeaways from our journey through the Gizmo:
- Temperature is a measure of the average kinetic energy of the particles in a substance. The hotter the substance, the faster the particles move.
- The state of matter (solid, liquid, or gas) depends on the amount of energy the particles possess. As temperature increases, particles move faster, causing transitions between states of matter: solid to liquid (melting), liquid to gas (evaporation), and so on.
- Particle motion is essential for understanding many real-world phenomena such as cooking, weather patterns, and even the operation of internal combustion engines.
Gizmo Temperature And Particle Motion Answers
The Ever-Expanding World of Particle Motion
The Gizmo is a tool that can open doors to a deeper understanding of the world around us. It allows us to see the invisible, providing a glimpse into the intricate world of particle motion. This understanding can empower us to make informed decisions about our world, from the energy we use to the materials we choose for our homes. So, let’s delve into this fascinating world of particles, and uncover the mysteries of their captivating movements!






