Have you ever wondered why a lemon tastes sour but baking soda tastes bitter? Or why some solutions bubble when mixed with vinegar while others stay calm? These are just a few examples of the fascinating world of acids and bases, a world where pH dictates the nature of a substance, influencing its properties, reactions, and even its role in our daily lives. Understanding acids, bases, and pH can be a bit daunting, but fear not! This guide will delve into the nitty-gritty of acids, bases, and pH, and unravel the mysteries behind those perplexing worksheet answers.
Image: blog.praxilabs.com
Imagine you’re in a chemistry class, a daunting worksheet filled with questions about acids, bases, and pH staring back at you. You might feel a pang of anxiety, unsure where to begin. But don’t fret! Understanding the essence of acids, bases, and pH is like unlocking a secret code that reveals the nature of our world. From the sourness of citrus fruits to the sting of vinegar, from the slippery feel of soap to the effervescence of antacids, these concepts are woven into the fabric of our everyday lives. This guide is your trusty companion, empowering you to conquer the complexities of acids, bases, and pH worksheets with confidence.
A Journey into the Heart of Acids and Bases
Let’s embark on a journey into the fundamental nature of acids and bases, unraveling the mysteries that often leave learners puzzled.
1. Defining the Players: Acids and Bases
In the realm of chemistry, acids and bases are like two sides of a coin, each possessing unique characteristics. Acids, often characterized by their sour taste (think lemon juice), are substances that release hydrogen ions (H+) when dissolved in water. This release of hydrogen ions makes the solution acidic. On the other hand, bases, often categorized by their bitter taste and slippery feel (think soap), produce hydroxide ions (OH-) when dissolved in water, turning the solution basic or alkaline.
2. The pH Scale: A Measure of Acidity and Basicity
Imagine a numerical scale that measures the acidity or basicity of a solution. This is precisely what the pH scale does. It’s a logarithmic scale ranging from 0 to 14, with 7 marking the neutral point. Solutions with a pH less than 7 are considered acidic, while those with a pH greater than 7 are basic or alkaline. The lower the pH value, the more acidic the solution, and vice versa.
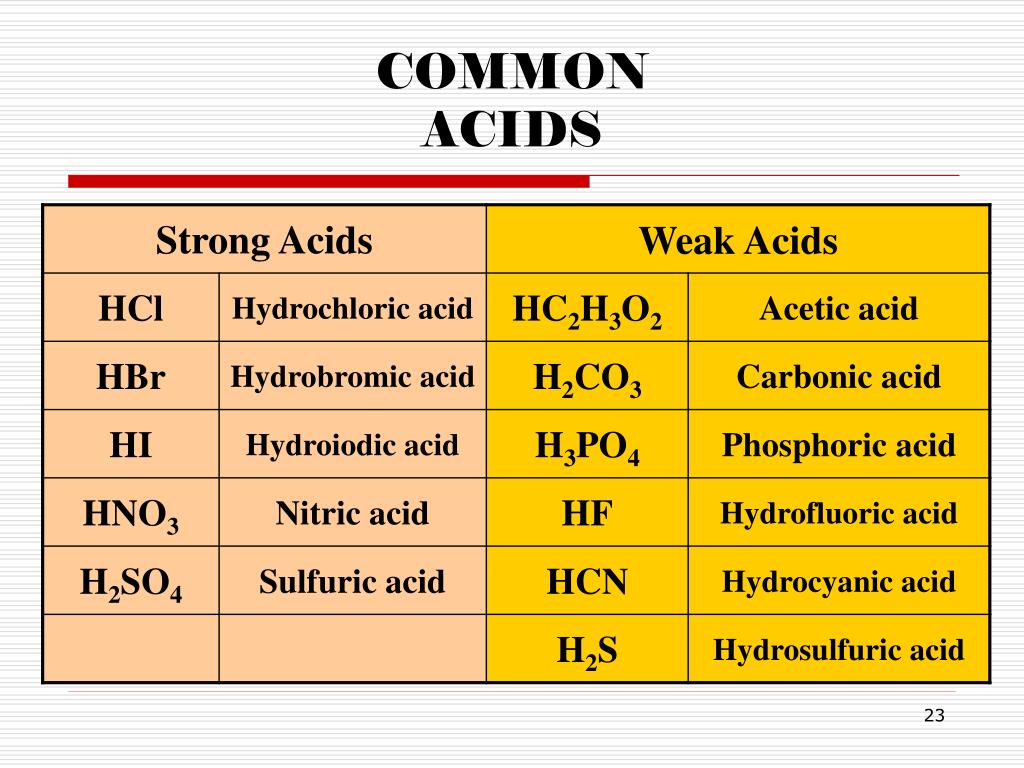
Image: fity.club
3. Understanding the pH Scale
- Highly acidic: A pH of 0 indicates the highest level of acidity, like concentrated hydrochloric acid.
- Neutral: Pure water has a pH of 7. It is neither acidic nor basic.
- Moderately acidic: Substances like lemon juice and vinegar have pH values ranging from 2 to 3.
- Slightly acidic: Most fruits have pH values between 3 and 4.
- Slightly basic: Baking soda, with a pH around 8, is slightly basic.
- Moderately basic: Soap, with a pH above 9, is moderately basic.
- Highly basic: Strong bases, like sodium hydroxide, have pH values around 14.
4. Key Indicators and Reactions
Certain indicators, like litmus paper, can help us visually identify the acidity or basicity of a solution. Litmus paper turns red in acidic solutions and blue in basic solutions.
Acids and bases react in a process called neutralization. This reaction involves the combination of hydrogen ions (H+) from an acid with hydroxide ions (OH-) from a base, producing water (H2O) and a salt.
5. The Importance of pH
The pH of a solution plays a crucial role in various aspects of our lives:
- Biological Processes: Our bodies maintain a delicate pH balance for proper functioning. Enzymes, which regulate vital chemical reactions, work best within specific pH ranges.
- Environmental Health: The pH of lakes, rivers, and oceans profoundly affects aquatic life. Acid rain, caused by the release of acidic pollutants, can disrupt ecosystems.
- Industrial Applications: The pH of solutions is vital in many industries, including food production, pharmaceutical manufacturing, and wastewater treatment.
Unraveling the Worksheet Answers: Practical Insights
Now that we’ve established the fundamentals, let’s dive into the practicalities of tackling those perplexing worksheet questions:
-
Identifying Acids and Bases: When faced with a list of substances, remember the key characteristics:
- Acids are sour, release H+ ions in water.
- Bases are bitter, slippery, release OH- ions in water.
-
Determining pH: Use pH scales or indicators (like litmus paper) to determine the pH of substances. Remember:
- A pH less than 7 indicates an acid.
- A pH greater than 7 indicates a base.
- A pH of 7 indicates a neutral solution.
-
Neutralization Reactions: When an acid reacts with a base, the reaction is called neutralization.
- The products of this reaction are water (H2O) and a salt.
- The pH of the resulting solution will generally be closer to 7 (neutral).
-
Common Examples:
- Fruits like lemons and oranges are acidic.
- Sodium bicarbonate (baking soda) is a base.
- Vinegar is acidic, often used to neutralize baking soda.
- Antacids are bases that neutralize stomach acids.
Acids Bases & Ph Worksheet Answers
Embracing the Power of Knowledge
Armed with this newfound understanding, you’ll be able to approach your acids, bases, and pH worksheets with confidence. Remember, the key is to understand the fundamental principles and apply them to real-world examples. Don’t hesitate to explore further resources, experiment with different substances, and ask questions. The world of chemistry, especially the fascinating realm of acids, bases, and pH, is full of exciting discoveries waiting to be made!






