Imagine looking at a beautiful orchestra, each instrument playing its unique melody, all working together in perfect harmony. Now, picture an atom, not as a tiny, solid ball, but as a miniature symphony, where electrons dance around the nucleus, each with its own specific energy and position. This intricate arrangement, known as electron configuration, is the very essence of an atom’s identity, dictating its chemical behavior and the properties of the matter it forms.

Image: www.pinterest.com
Understanding electron configuration is like unlocking the secrets of the periodic table, a chart that organizes elements based on their recurring properties. Each element has a unique electron configuration, revealing its place within the grand symphony of chemistry. In this exploration, we will delve into the captivating world of electron configurations, deciphering the language of atoms and unlocking the secrets behind their astonishing diversity.
A Symphony of Electrons: Writing the Atomic Score
The electron configuration of an element lays out the specific arrangement of its electrons in different energy levels and sublevels. Think of it like a map of an atom’s internal structure, revealing the electrons’ dance around the nucleus. Each energy level, represented by a number, corresponds to a distinct region of space around the nucleus, with higher numbers signifying greater distances. Within each energy level, electrons occupy sublevels, designated by letters (s, p, d, f), each with its own distinct shape and energy.
The foundation for understanding electron configurations lies in the quantum mechanical model of the atom, a departure from the classical view of electrons orbiting the nucleus like tiny planets. This model proposes that electrons exist in specific energy states, described by a set of four quantum numbers:
- Principal Quantum Number (n): Indicates the energy level, with higher numbers corresponding to higher energy levels. For example, n=1 represents the first energy level, n=2 the second, and so on.
- Angular Momentum or Azimuthal Quantum Number (l): Determines the shape of an electron orbital, taking values from 0 to n-1. l=0 corresponds to an s orbital (spherical shape), l=1 to a p orbital (dumbbell shape), l=2 to a d orbital (more complex shapes), and l=3 to an f orbital (even more intricate shapes).
- Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space, taking values from -l to +l, including 0. For example, a p orbital (l=1) has three possible orientations in space (ml=-1, 0, +1).
- Spin Quantum Number (ms): Describes the intrinsic angular momentum of an electron, suggesting it is spinning, which generates a magnetic field. It can have two values, +1/2 or -1/2, representing opposite spin orientations.
Decoding the Symphony: Building Electron Configurations
Imagine you have a set of building blocks, each representing an electron. You start with the lowest energy level (n=1) and fill the blocks according to specific rules:
- Aufbau Principle: Electrons fill orbitals in increasing order of energy. This principle dictates the sequence of orbital filling, starting with the lowest energy orbitals and gradually ascending to higher energy ones.
- Hund’s Rule: Electrons fill orbitals individually before pairing up within the same sublevel. This rule ensures that each orbital within a sublevel is occupied by a single electron before any pairing occurs.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, with opposite spins. This principle prevents two electrons in the same atom from having the same set of four quantum numbers, ensuring a unique “address” for each electron.
To write the electron configuration of an element, you start with the element’s atomic number, which represents the number of protons (and hence electrons) in its atom. Then, follow these steps:
- Identify the energy levels: The principal quantum number (n) indicates the energy levels. Start with n=1, then n=2, and so on.
- Determine the sublevels: Within each energy level, identify the sublevels (s, p, d, f).
- Fill the orbitals: Follow the Aufbau principle and Hund’s rule to fill the orbitals with electrons. Remember that each orbital can hold a maximum of two electrons with opposite spins (Pauli exclusion principle).
For example, consider the element nitrogen (atomic number 7). It has 7 electrons. Using the above steps, we get the following electron configuration:
1s² 2s² 2p³
This notation signifies that nitrogen has two electrons in its 1s orbital, two electrons in its 2s orbital, and three electrons in its 2p orbitals.
The Periodic Table: A Symphony of Patterns
Electron configuration reveals a profound connection between the elements and their position on the periodic table. The periodic table is not just a random arrangement; it reflects the underlying structure of electron configuration.
- Periods: Rows on the periodic table correspond to the principal quantum number (n), representing energy levels. Thus, the first period contains elements with n=1, the second period with n=2, and so on.
- Groups: Columns on the periodic table represent elements with similar electron configurations, particularly the number of valence electrons, those in the outermost energy level. Valence electrons are crucial for chemical bonding as they dictate how elements interact with each other.
The electron configurations of elements in the same group exhibit similar patterns in their outermost energy levels. For instance, all elements in Group 1 (alkali metals) have one electron in their outermost energy level, while all elements in Group 17 (halogens) have seven electrons in their outermost energy level. This shared characteristic is reflected in their similar chemical properties, making the periodic table a powerful tool for understanding the behavior of elements.
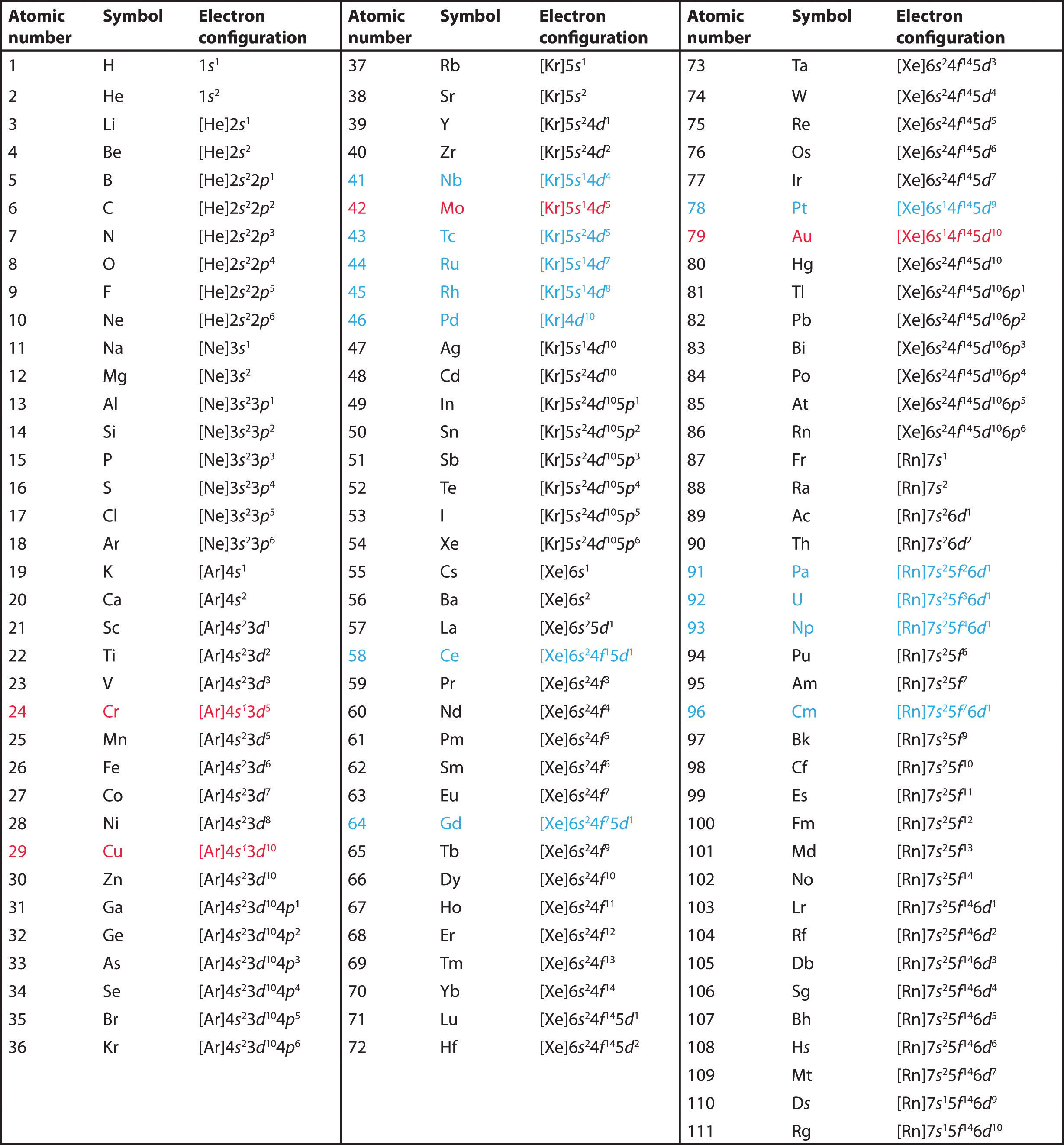
Image: chem.libretexts.org
Beyond the Basics: Unveiling the Complexity
While the basic principles of electron configuration are relatively straightforward, the world of atoms is far more intricate, leading to exceptions and complexities.
-
Exceptions to the Aufbau Principle: While the Aufbau principle generally guides electron configuration, certain elements deviate from this rule due to the subtle interplay of energy levels and the influence of electron-electron interactions. These exceptions are often related to the stability associated with half-filled or completely filled orbitals. For example, copper (Cu) has one electron in the 4s orbital instead of two in the 3d orbital.
-
Transition Metals: Transition metals, located in Groups 3-12 of the periodic table, display unique electron configurations due to the close proximity of their d orbitals. This proximity can result in multiple possible electron configurations for the same element, leading to complex magnetic properties and diverse chemical behavior.
-
Lanthanides and Actinides: Elements in the lanthanide and actinide series feature complex electron configurations, particularly with the filling of f orbitals, which are even more numerous and contribute to the unique characteristics of these elements.
Electron Configuration in Action: Shaping Our World
Beyond the theoretical realm, electron configuration plays a pivotal role in shaping the world around us. It determines the properties of materials, governs chemical reactions, and underpins technologies that make our lives easier.
-
Chemical Reactivity: The number and arrangement of valence electrons determine an element’s reactivity, whether it readily forms bonds with other elements or remains stable on its own. Understanding electron configurations allows us to predict chemical reactions and design new materials with specific properties.
-
Material Properties: The electron configurations of atoms determine the properties of materials, such as electrical conductivity, thermal conductivity, and magnetic behavior. For example, metals generally are good conductors of electricity because their valence electrons are loosely held and can move freely.
-
Technological Advancements: The insights from electron configurations have led to breakthroughs in various fields, from developing new catalysts for chemical reactions to designing high-performance semiconductors for electronics.
Electron Configuration Of All The Elements
https://youtube.com/watch?v=_16gaEi5K_U
Mastering the Symphony: A Guide to Further Exploration
Learning about electron configuration is a journey into the heart of chemistry, offering valuable insights into the nature of matter. Here are some actionable tips for further exploration:
- Practice: Write out the electron configurations of different elements to solidify your understanding. Use online tools or resources to check your accuracy.
- Explore the Periodic Table: Examine the periodic table and observe the patterns in electron configurations across periods and groups. This visual representation can enhance your understanding of element behavior.
- Connect Theory to Applications: Seek examples of how electron configurations impact our world, from manufacturing novel materials to developing cutting-edge technologies.
Electron configurations are not just theoretical concepts; they are the building blocks of our world, shaping the properties of matter, influencing chemical reactions, and driving technological advancements. By understanding electron configurations, we unlock a deeper appreciation for the complex and fascinating world of atoms and their role in shaping our lives. So, delve into the fascinating world of electron configurations, and discover the symphony of electrons that orchestrates the universe around us.






