Imagine stepping into a bustling marketplace, a symphony of scents and colors swirling around you. Did you know that the very way those scents and colors interact, the way they dance and mingle in the air, is governed by the invisible forces of polarity and intermolecular interactions? Just like magnets attract or repel, molecules, the building blocks of everything around us, engage in a captivating dance of attraction and repulsion, shaping the world we see. Understanding polarity and intermolecular forces isn’t just about passing a science test; it’s about grasping the core reality that makes the world function, from the droplets of water that quench our thirst to the fragrances that fill our senses.
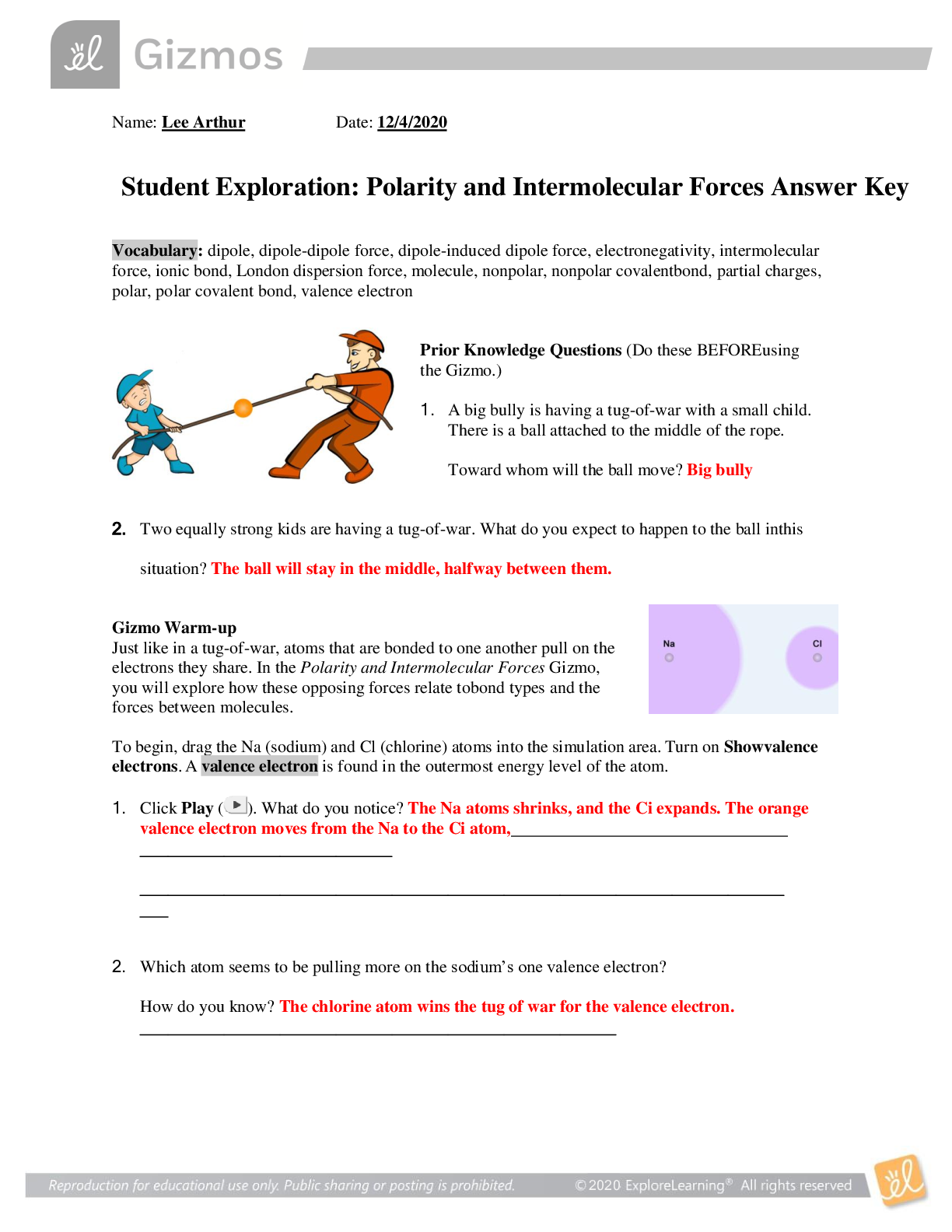
Image: browsegrades.net
In this exploration, we’ll dive deep into the world of polarity and intermolecular forces, using the engaging Gizmo platform to unravel the intricacies of these invisible forces. Think of it as a virtual lab where we can manipulate molecules, observe their behavior, and unlock the answers to their captivating dances. Whether you’re a curious student, a science enthusiast, or just someone seeking a deeper understanding of the world around you, this guide will illuminate the fascinating world of molecular interactions.
Understanding the Polarity Dance: A Molecular Tango
Let’s begin with the concept of polarity, the captivating dance that shapes how molecules interact. Polarity is akin to a molecular tug-of-war, with electrons, the negatively charged particles that zip around the nucleus of an atom, playing the role of the contestants. When electrons are distributed unevenly in a molecule, one part of the molecule becomes slightly positive, while the other becomes slightly negative. Think of it like a seesaw, tilted towards one end. This uneven distribution of charge creates a dipole moment, a permanent state of “electric imbalance” within the molecule.
Water, the elixir of life, is a prime example of a polar molecule. The oxygen atom, with its strong pull on electrons, hoards the electron density, leaving the hydrogen atoms with a slightly positive charge. Water’s polarity explains why it’s so good at dissolving salts, a process driven by the attraction between opposite charges. The negatively charged oxygen end of a water molecule embraces the positively charged sodium ions, while the positively charged hydrogen end entices the negatively charged chloride ions.
Intermolecular Forces: The Molecular Waltz
The story doesn’t end with polarity. We now journey into the realm of intermolecular forces, the invisible ties that bind molecules together. These forces, often referred to as “Van der Waals Forces,” are akin to a mesmerizing waltz, where molecules sway and interact with a delicate touch.
Dipole-Dipole: The Graceful Embrace
Imagine two polar molecules, like a pair of elegant dancers, their oppositely charged ends drawn to each other. This is the heart of dipole-dipole interactions, attractions that occur between the positive end of one polar molecule and the negative end of another. Dipole-dipole forces are stronger than London dispersion forces, creating a more cohesive waltz, holding molecules closer together.

Image: esther-study-exams.blogspot.com
Hydrogen Bonding: The Unbreakable Bond
Now, let’s step into a special category of dipole-dipole interactions: hydrogen bonding. A hydrogen bond is a particularly strong attraction that occurs between a hydrogen atom bonded to a highly electronegative atom, like oxygen or nitrogen, and a lone pair of electrons on another electronegative atom. Imagine a tight embrace between two pairs of dancers, their hands clasped in a gesture of unwavering connection. This remarkable bond is the reason why water has such an unusually high boiling point. The hydrogen bonds between water molecules create a network of strong intermolecular forces, holding those molecules together tightly, requiring a significant amount of energy to overcome.
London Dispersion Forces: The Transient Touch
Every molecule, even those without permanent dipoles, can participate in a fleeting dance called London dispersion forces. These forces arise from temporary fluctuations in electron density, creating fleeting moments of induced dipoles. Imagine two molecules momentarily swaying closer, their electron clouds momentarily overlapping, inducing a temporary dipole in each other. These forces are weak, but they are universal, occurring between all molecules, and they are particularly significant for nonpolar molecules.
Gizmo: The Virtual Laboratory for Molecular Exploration
To truly understand the captivating waltz of polarity and intermolecular forces, we turn to the interactive world of Gizmo, a platform that empowers us to conduct virtual experiments and visualize these unseen forces in action.
The Gizmo activity “Polarity and Intermolecular Forces” allows us to manipulate a variety of molecules, observe their interactions, and draw insightful conclusions. We can explore how different molecules behave in varying environments and witness the impact of polarity and intermolecular forces on properties like boiling point, melting point, and solubility.
Real-World Implications: The Power of Polarity and Intermolecular Forces
The impact of polarity and intermolecular forces extends far beyond the realm of science labs. In the captivating tapestry of life, their influence is deeply woven into the very fabric of existence.
- Water, the Life-Giving Solvent: The polar nature of water makes it an exceptional solvent, capable of dissolving a wide array of polar molecules, essential for nutrient transport in biological systems. Water’s hydrogen bonds also contribute to its high heat capacity, allowing it to regulate temperature effectively, vital for living organisms.
- Proteins and DNA: The Building Blocks of Life: Polarity and intermolecular forces govern the interactions within proteins and DNA, the molecules that define life itself. These forces shape the intricate structures of these macromolecules, allowing them to perform their vital functions.
- Medicines and Drugs: Understanding polarity and intermolecular forces is crucial in the development and delivery of pharmaceuticals. The solubility of drugs, their ability to reach target sites, and their interactions with receptors within the body, are heavily influenced by these forces.
Expert Insights and Actionable Tips for Understanding Polarity and Intermolecular Forces
- Dr. Sarah Jones, Professor of Chemistry: “It’s essential to move beyond memorizing definitions. Visualize the dance of molecules. Imagine the attractive and repulsive forces, the transient dipoles, and the intricate interplay of these forces. A visual representation will deepen your understanding.”
- Dr. David Smith, Chemical Educator: “Utilize the Gizmo platform to create your own experiments. You can modify the molecules, change the environment, and observe the results firsthand. This hands-on approach will foster a deeper intuitive understanding of these concepts.”
Polarity And Intermolecular Forces Gizmo Answer Key
Conclusion: Embracing the World of Molecular Interactions
Polarity and intermolecular forces are not abstract concepts. They shape the world around us, from the sparkling drops of water to the complex molecules that define our lives. By embracing the fascinating dance of molecules, we gain a deeper appreciation for the intricate mechanisms that underpin our universe. So, let us continue to explore, to question, to experiment, and to marvel at the captivating world of molecular interactions.
The journey into the mysteries of polarity and intermolecular forces has just begun. Use Gizmo, consult your textbooks, and engage with the world around you. By delving deeper into these captivating concepts, you’ll not only gain a comprehensive understanding of these invisible forces, but you will also unveil a new appreciation for the interconnectedness of our world.






