Have you ever wondered how the world around us works at its most fundamental level? From the rusting of a metal object to the fizz of an effervescent tablet in your drink, chemical reactions are the driving force behind countless phenomena that shape our daily lives. Understanding these reactions and the principles governing them is crucial for anyone seeking to grasp the intricacies of chemistry. This guide will delve into the vital concepts covered in Chemistry Unit 7 Worksheet 3, providing you with a clear path to mastering the art of chemical transformations.
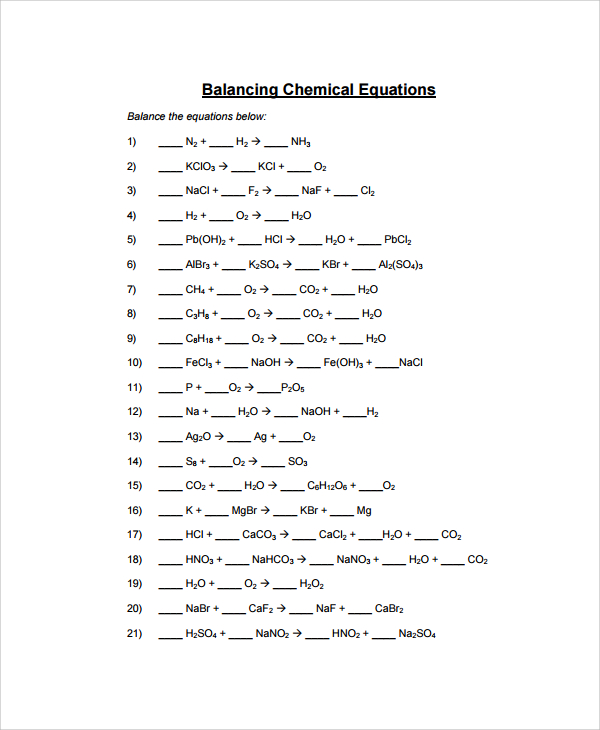
Image: www.sampletemplates.com
This worksheet, often a crucial stepping stone in any chemistry curriculum, is designed to reinforce your understanding of chemical reactions and their characteristics. It bridges the gap between theoretical concepts and practical application, allowing you to delve into the fascinating world of reacting molecules and the energy transformations they entail. By the end of this guide, not only will you be able to tackle the questions on Worksheet 3 with confidence, but you will also gain a deeper appreciation for the foundational principles that govern the chemical universe.
Delving into the Realm of Chemical Reactions: A Primer
Unraveling the Fundamentals of Chemical Reactions
At its core, a chemical reaction involves the rearrangement of atoms and molecules, resulting in the formation of new substances with distinct properties. Think of it as a molecular dance, where reactants, the starting materials, intertwine and rearrange to create products, the resulting substances. Imagine baking a cake—you begin with flour, sugar, eggs, and butter (reactants) and through the process of mixing, baking, and cooling, you transform these ingredients into a delectable cake (product), a completely different entity than its initial components!
Types of Chemical Reactions: A Colorful Tapestry of Transformation
The world of chemical reactions is brimming with diversity. Imagine a bustling marketplace, where different vendors offer a unique array of goods. Similarly, we categorize chemical reactions into different types based on their distinct characteristics:
- Combination Reactions: In this type of reaction, two or more substances combine to form a single, more complex product. Imagine combining two building blocks to create a single, larger structure. For example, the burning of magnesium in air—2Mg (magnesium) + O2 (oxygen) → 2MgO (magnesium oxide).
- Decomposition Reactions: These reactions are the inverse of combination reactions, where a single compound breaks down into two or more simpler substances. Think of dismantling a complex structure into its individual components. For instance, the decomposition of calcium carbonate upon heating—CaCO3 (calcium carbonate) → CaO (calcium oxide) + CO2 (carbon dioxide).
- Single Displacement Reactions: In these reactions, a more reactive element displaces a less reactive one from a compound. Picture a strong athlete pushing a weaker one aside to take their position. For example, zinc reacting with hydrochloric acid—Zn (zinc) + 2HCl (hydrochloric acid) → ZnCl2 (zinc chloride) + H2 (hydrogen gas).
- Double Displacement Reactions: Two compounds switch partners, resulting in the formation of two new compounds with different combinations of ions. Like a lively dance where partners change, but the total number of dancers remains the same. For example, silver nitrate reacting with sodium chloride—AgNO3 (silver nitrate) + NaCl (sodium chloride) → AgCl (silver chloride) + NaNO3 (sodium nitrate).

Image: quizzlibraryzimmer.z13.web.core.windows.net
Balancing the Equations: The Art of Conservation
The key to understanding any chemical reaction lies in the ability to balance its equation. This process ensures that the number of atoms of each element remains constant on both sides of the equation, upholding the fundamental law of conservation of mass. Imagine a perfectly balanced scale—the total mass on one side must always equal the total mass on the other. In balancing equations, we adjust the coefficients in front of each chemical formula to achieve this balance. For example, the reaction between hydrogen and oxygen to form water can be balanced as follows: 2H2 + O2 → 2H2O.
Navigating Chemistry Unit 7 Worksheet 3: A Guided Walkthrough
Question 1: Identification of Reactants and Products
This question assesses your ability to identify the starting materials (reactants) and the resulting substances (products) in a given chemical reaction. Think of it as recognizing the ingredients used in a recipe and the final dish prepared. For example, in the reaction: Fe (iron) + S (sulfur) → FeS (iron sulfide), iron and sulfur are the reactants, while iron sulfide is the product.
Question 2: Classifying Chemical Reactions
This question tests your knowledge of the different types of chemical reactions we discussed earlier. Like a detective piecing together clues to solve a mystery, you need to analyze the equation and determine which category it belongs to. For instance, the reaction: 2Na (sodium) + Cl2 (chlorine) → 2NaCl (sodium chloride) is a combination reaction, as two elements combine to form a single compound.
Question 3: Balancing Chemical Equations
This question requires you to apply the principles of conservation of mass and adjust the coefficients in the chemical equation to ensure that the number of atoms for each element is equal on both sides of the equation. Think of it as finding the perfect balance between the scales of reactants and products. For example, the equation: CH4 (methane) + O2 (oxygen) → CO2 (carbon dioxide) + H2O (water) needs balancing to ensure the number of carbon, hydrogen, and oxygen atoms is the same on both sides: CH4 + 2O2 → CO2 + 2H2O.
Question 4: Predicting Products of Chemical Reactions
This question goes beyond simply recognizing types of reactions and requires you to predict the likely products based on the reactants and their known reactivity. Think of it as playing a chemical puzzle game, where you use your knowledge of reactants and their tendencies to deduce the likely outcome. For instance, given the reaction: AgNO3 (silver nitrate) + NaCl (sodium chloride) → X (unknown product), you can predict the product to be AgCl (silver chloride) based on the general knowledge that silver ions form an insoluble compound with chloride ions.
Question 5: Applying Chemical Equations to Real-World Scenarios
This question demonstrates the practical relevance of chemical reactions by applying the concepts you’ve learned to real-world situations. Imagine using your knowledge of chemical reactions to understand various processes around you. For example, understanding the combustion reaction of fuels (hydrocarbons) is essential for understanding how cars engines operate.
Beyond the Worksheet: Exploring the World of Chemistry
Chemistry Unit 7 Worksheet 3 provides a solid foundation for understanding fundamental chemical reactions. But the learning doesn’t stop there. The world around us is a vast laboratory where countless chemical reactions occur every moment. Consider these examples:
- Photosynthesis: The process by which plants convert sunlight, water, and carbon dioxide into glucose (sugar) and oxygen is a complex series of chemical reactions that fuels life on Earth.
- Respiration: Our bodies use oxygen to break down glucose in a process called respiration, releasing energy to power our bodies and producing carbon dioxide and water as waste products.
- Pharmaceuticals: The development of new drugs and medicines involves understanding and manipulating chemical reactions to synthesize molecules with desired therapeutic properties.
- Material Science: From the development of new plastics to the creation of advanced composites, chemical reactions play a pivotal role in shaping the materials that define our world.
Chemistry Unit 7 Worksheet 3 Answer Key
Conclusion
Unlocking the secrets of Chemistry Unit 7 Worksheet 3 goes beyond simply completing the exercises. It involves delving into the fundamentals of chemical reactions, understanding their diverse types, and appreciating the intricate dance of atoms and molecules that govern these transformations. As you navigate through the world of chemical reactions, remember that every seemingly simple event around you, from the rusting of iron to the baking of a cake, is a testament to the power and elegance of chemistry. So, continue your exploration, delve deeper into the fascinating world of chemical transformations, and watch as your understanding of the world around you grows exponentially!






