Imagine you’re a scientist working in a cutting-edge laboratory, tasked with designing a new type of rocket fuel. Your success hinges on understanding how gases behave under extreme pressure and temperature. This is where the fascinating world of gas laws comes in. These fundamental principles, governing the behavior of gases, are essential not only for rocket engineers but also for everyday applications like cooking, weather forecasting, and even scuba diving. But how can you grasp these laws without the expensive and potentially dangerous equipment used in laboratories? The answer lies in the captivating world of PHET simulations.
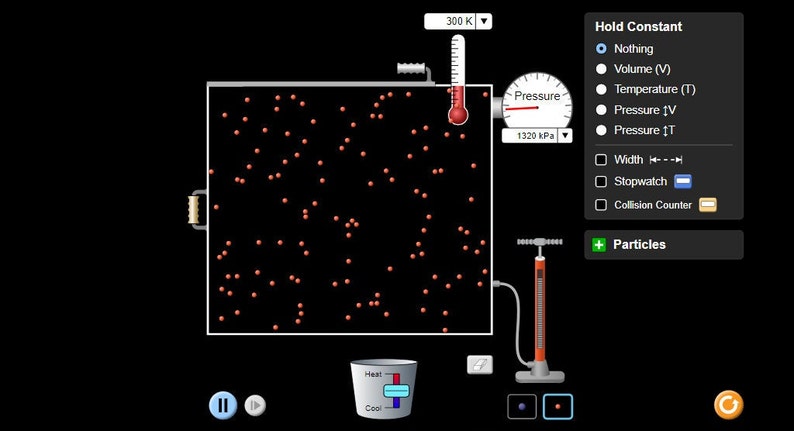
Image: www.etsy.com
The PHET simulations, developed by the University of Colorado Boulder, offer an interactive and engaging way to explore scientific concepts, including the fundamental laws of gas behavior. PHET’s Gas Laws simulation, in particular, provides a virtual laboratory where you can experiment with different variables, such as pressure, temperature, and volume, to observe their impact on ideal gases. This hands-on experience, accessible from the comfort of your home or classroom, allows you to dive deep into the complex world of gas behavior without any need for costly or dangerous equipment. This article will serve as your guide, leading you through the fascinating intricacies of the PHET Gas Laws simulation, unraveling the secrets of each gas law and unlocking the key to understanding their real-world applications.
Delving into the Gas Laws
The PHET Gas Laws simulation is a treasure trove of knowledge, offering a platform to explore four fundamental gas laws: Boyle’s Law, Charles’s Law, Gay-Lussac’s Law, and Avogadro’s Law. Let’s embark on a journey to understand each of these laws individually, exploring their significance and the insights the PHET simulation provides.
Boyle’s Law: The Inverse Relationship
Boyle’s Law, a cornerstone of gas behavior, states that the volume of a gas is inversely proportional to its pressure, provided the temperature and the amount of gas remain constant. Imagine a balloon filled with air. As you increase the pressure on the balloon, its volume decreases; conversely, if you decrease the pressure, the balloon expands. This inverse relationship is visually represented in the PHET simulation.
The simulation allows you to manipulate the pressure on a sealed container containing gas, observing the corresponding change in volume. By moving a piston, you can increase or decrease the pressure, and the simulation instantly reflects the resulting volume change. This interactive experience provides a unique opportunity to see Boyle’s Law in action and grasp the concept intuitively.
Charles’s Law: The Direct Proportionality
Next on our journey is Charles’s Law, which states that the volume of a gas is directly proportional to its temperature, assuming the pressure and the amount of gas remain constant. Imagine a hot air balloon. As the air inside the balloon is heated by a burner, its volume expands, causing the balloon to rise. This direct relationship between temperature and volume is brought to life in the PHET simulation.
Within the simulation, you can alter the temperature of the gas in a sealed container, either by heating or cooling it. The simulation then dynamically displays the associated volume change, vividly demonstrating how the volume increases as the gas gets hotter and decreases as it cools down. This interactive experience allows you to visualize and comprehend the relationship between temperature and volume with ease.

Image: inspirenetic.blogspot.com
Gay-Lussac’s Law: Pressure & Temperature Hand in Hand
Gay-Lussac’s Law delves into the relationship between pressure and temperature in a gas, stating that the pressure of a gas is directly proportional to its temperature, provided the volume and the amount of gas remain constant. Think about a pressure cooker. As you increase the temperature of the food within the cooker, the pressure inside also increases. This direct relationship between pressure and temperature is brought to life within the PHET simulation.
The simulation allows you to manipulate the temperature of a sealed container of gas, visually demonstrating the corresponding change in pressure. This interactive process allows you to grasp the concept intuitively, seeing how the pressure increases as the gas gets hotter and decreases as it cools down.
Avogadro’s Law: Unveiling the Relationship Between Moles and Volume
Avogadro’s Law focuses on the relationship between the number of moles of gas and the volume, stating that the volume of a gas is directly proportional to the number of moles of gas (or the number of molecules), assuming the temperature and pressure remain constant. Imagine a container filled with a fixed amount of gas. As you add more gas molecules to the container, the volume will increase proportionally.
The PHET simulation allows you to experiment by adding or removing gas molecules from a sealed container. You can observe how the volume changes in direct proportion to the number of molecules present, giving you a visual understanding of this crucial gas law.
Beyond the Basics: Combining the Gas Laws
The PHET simulation doesn’t stop at individual gas laws; it allows you to explore the combined gas law, which encompasses the relationships between pressure, volume, and temperature simultaneously. You can experiment with different combinations of these variables, observing their combined effect on the gas. This interactive approach allows you to unravel the complex interplay between these factors, gaining a deeper understanding of how gases behave under changing conditions.
Imagine a scuba diver descending into the ocean depths. As the diver goes deeper, the pressure surrounding them increases, causing the volume of the air in their lungs to decrease. This complex scenario is perfectly captured in the PHET simulation, allowing you to observe the effects of pressure and volume changes on a gas simultaneously.
Real-World Applications: Gas Laws in Action
The knowledge gained from exploring the PHET Gas Laws simulation extends far beyond the confines of a virtual lab. It has widespread practical applications across various industries and aspects of daily life.
Meteorology: Predicting the Weather
Weather forecasting relies heavily on understanding gas laws. Meteorologists use these laws to predict temperature, pressure, and wind changes, ultimately helping them to forecast storms, thunderstorms, and other weather phenomena.
Aerospace Engineering: Powering Rockets
Rocket scientists use gas laws to design efficient engines that generate thrust by controlled combustion of rocket fuel, a process that involves understanding the behavior of gases under extreme conditions.
Medical Applications: Respiration and Anesthesia
Gas laws play a crucial role in understanding respiration and anesthesia. The exchange of oxygen and carbon dioxide in the lungs follows principles of gas pressure and volume, while medical professionals use gas laws to calculate the dosages of anesthetic gases.
Industrial Applications: Manufacturing and Chemical Processes
Gas laws are fundamental to various industrial applications, including manufacturing and chemical processes. Controlling the temperature, pressure, and volume of gases is essential for optimizing production efficiency and ensuring product quality.
Expert Insights and Actionable Tips
Harnessing the power of the PHET Gas Laws simulation is like having a world-class science lab at your fingertips. To make the most of this invaluable resource, consider these expert-backed tips:
-
Start with the Basics: Begin your exploration by focusing on the individual gas laws, such as Boyle’s Law or Charles’s Law. This will provide a solid foundation for understanding the more complex relationships between different gas variables.
-
Experiment and Observe: The beauty of the PHET simulation lies in its interactive nature. Experiment with different combinations of pressure, volume, and temperature, carefully observing the resulting changes in the gas.
-
Visualizing the Concepts: Don’t just read about gas laws; visualize them! Try drawing diagrams or creating mental models to represent the relationships between different gas parameters.
-
Practical Applications: Look for real-world examples of gas laws in action, such as weather patterns, cooking, or even the operation of your car engine. This will help you see how these abstract concepts apply to everyday life.
Gas Laws Phet Simulation Answer Key
Key Takeaways: A Final Word
The PHET Gas Laws simulation serves as a gateway to a captivating world of scientific exploration. By engaging with this interactive tool, you can unlock the secrets of gas behavior, gaining a deeper understanding of these fundamental principles and their real-world applications. Remember to experiment, visualize, and explore the fascinating world of gas laws, a world that holds the key to understanding everything from weather patterns to rocket propulsion. So, dive into the PHET Gas Laws simulation and embark on a journey of scientific discovery!
Don’t stop here! Share your experiences exploring the PHET simulation with others, and explore additional resources online to deepen your understanding of gas laws. This journey of knowledge is just beginning!






