Imagine yourself as a tiny atom, adrift in a vast sea of other atoms. You feel a tug, a pull, a desperate yearning for connection. This is the story of chemical bonding, the invisible force that holds the universe together, from the air we breathe to the water we drink. It’s a story of attraction, repulsion, and the delicate dance of electrons that creates the molecules that make up everything around us. And it’s a story that unfolds beautifully in the realm of ionic and covalent worksheets.
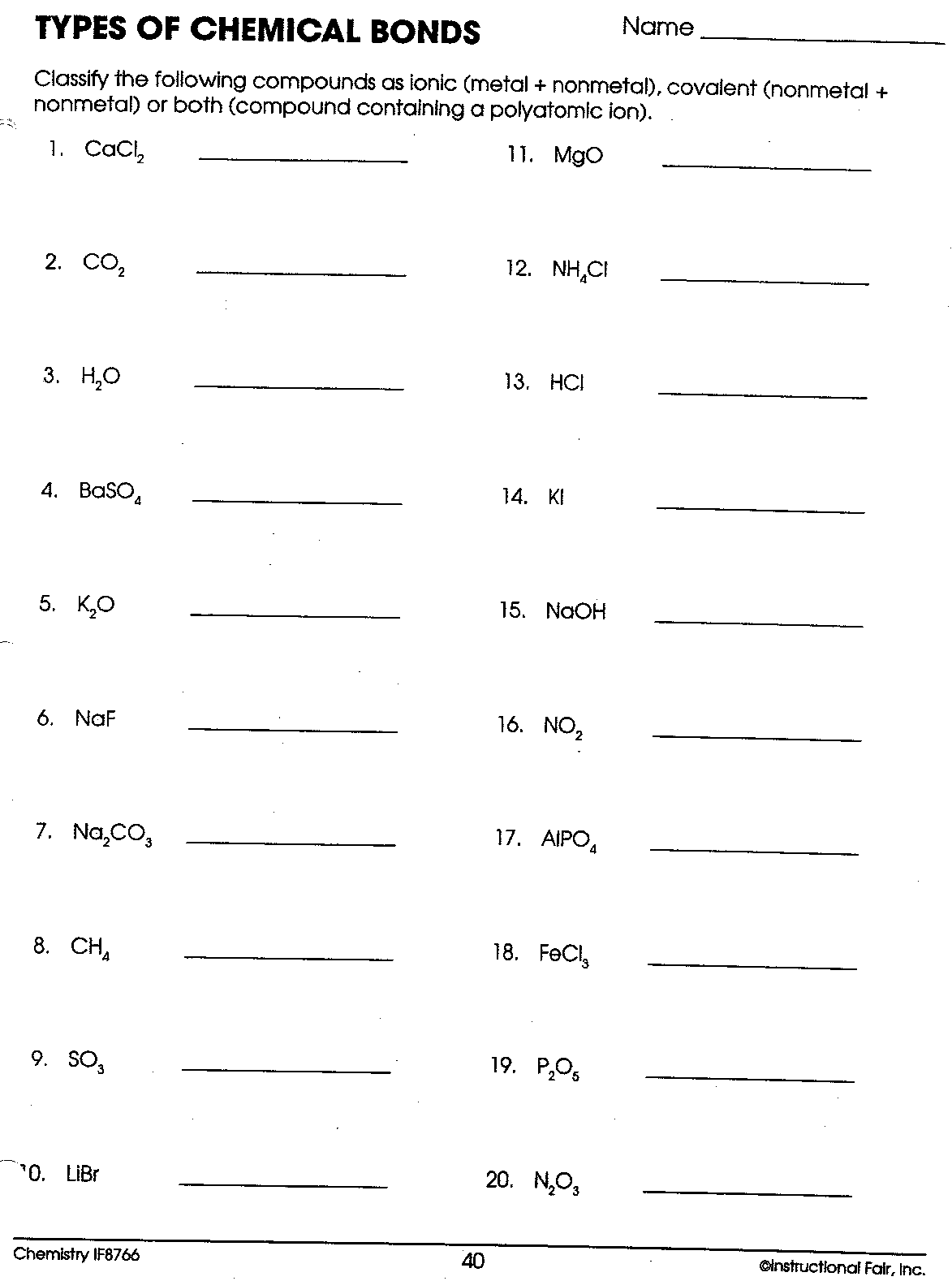
Image: printablejacob.z22.web.core.windows.net
These worksheets are more than just exercises; they are gateways into the fascinating world of chemical bonds, offering a practical and interactive way to understand the fundamental principles that govern the interactions between atoms. They can be found in classrooms, labs, and homes, serving as invaluable tools for students, educators, and anyone curious about the building blocks of our universe.
The Basics of Chemical Bonding: Where It All Begins
At its core, chemical bonding is all about achieving stability. Atoms, with their energetic electrons whizzing around the nucleus, are constantly seeking a balance, a state of lower energy. They accomplish this by sharing or transferring electrons with other atoms, forming stable molecules.
There are two primary types of chemical bonds: ionic and covalent.
Ionic Bonds: The Dance of Opposites
Ionic bonds are a bit like a love story between opposites. They arise when one atom, typically a metal, generously donates one or more electrons to another atom, usually a nonmetal. This exchange of electrons creates charged particles called ions: a positively charged cation and a negatively charged anion. Their opposite charges then pull them together, forming a strong electrostatic bond.
Imagine a dance floor with a group of enthusiastic metal atoms and a group of shy nonmetal atoms. The metal atoms, always eager to share, offer their electrons to the more reserved nonmetal atoms. This exchange creates an irresistible attraction between them, leading to a dance that’s both passionate and stable.
Covalent Bonds: Sharing is Caring
Covalent bonds, on the other hand, are all about sharing. Two or more nonmetal atoms, often with a similar thirst for electrons, decide to pool their resources and share electrons. They create a bond by overlapping their electron orbitals, effectively sharing electrons and achieving a state of mutual contentment.
Think of a group of friends holding hands, each contributing their own fingers to make a stronger, more stable connection. This act of sharing is the essence of covalent bonding, where atoms come together to form a unified whole, benefiting from the combined strength of their shared resources.

Image: www.pinterest.se
Ionic and Covalent Worksheets: Your Gateway to Understanding
Now, let’s talk about the tools that allow us to delve deeper into the complexities of ionic and covalent bonds: worksheets. These invaluable resources offer a hands-on approach, transforming the abstract concepts of chemical bonding into tangible, interactive experiences.
Unlocking the Secrets of Ionic Bonds
Ionic bonding worksheets often involve identifying the charges of ions, predicting the formulas of ionic compounds, and drawing Lewis structures. They might present a list of elements and their electronegativity values, encouraging students to calculate the difference in electronegativity and determine the type of bond formed. Other worksheets might require students to write balanced chemical equations for ionic reactions, understanding the role of oxidation and reduction.
Exploring the Wonders of Covalent Bonds
Covalent bonding worksheets offer a similar range of challenges. They might ask students to draw Lewis structures of various covalent molecules, identifying the number of shared electrons and the bond order. They might also delve into the concept of hybridization, examining the shapes of molecules and predicting their polarity.
These worksheets provide a structured environment for students to practice what they’ve learned. They encourage them to think critically about the bonding process, applying the concepts they’ve acquired to real-world examples.
Beyond the Worksheet: Real-World Applications of Chemical Bonding
Chemical bonding isn’t confined to the pages of textbooks and worksheets; it’s the very foundation of our world.
The Power of Ionic Bonds
Ionic bonds are the backbone of many materials we encounter daily. Table salt, a classic example, is held together by ionic bonds between sodium and chloride ions. These bonds also play a crucial role in the formation of crystals, like the dazzling diamonds that mesmerize us with their brilliance.
The Versatility of Covalent Bonds
Covalent bonds are responsible for the formation of countless molecules that sustain life, including the water we drink, the air we breathe, and the food we eat. Our DNA, the blueprint of life, is held together by covalent bonds between nucleotides. Polymers, like plastics and fabrics, are also formed by chains of covalently bonded monomers.
Expert Insights for Mastering Chemical Bonding
To truly grasp the principles of chemical bonding, it’s crucial to seek guidance from experts in the field.
“The key to understanding chemical bonding is to visualize the interactions between atoms,” says Dr. Emily Carter, a renowned chemist specializing in computational materials science. “Imagine the dance of electrons, the attraction and repulsion of charges, and the continuous movement that defines the nature of bonds.”
“Don’t be afraid to experiment,” advises Dr. Michael Faraday, a pioneer in the field of electrochemistry. “Use models, build structures, and play with different configurations to gain a deeper understanding of the concept.”
Chemical Bonding Ionic & Covalent Worksheet
Transforming Knowledge into Action: Your Journey Ahead
With the right tools and expert guidance, mastering chemical bonding isn’t just about passing exams; it’s about gaining a deeper appreciation for the invisible forces that shape our world.
So, grab a worksheet, explore the world of chemical bonding, and discover the magic of attraction, repulsion, and the delicate dance of electrons that binds everything together. Share your newfound knowledge with others, spark their curiosity, and together, let’s unveil the wonders of the universe, atom by atom.






